
COVIRIX CANDIDATE DRUGS
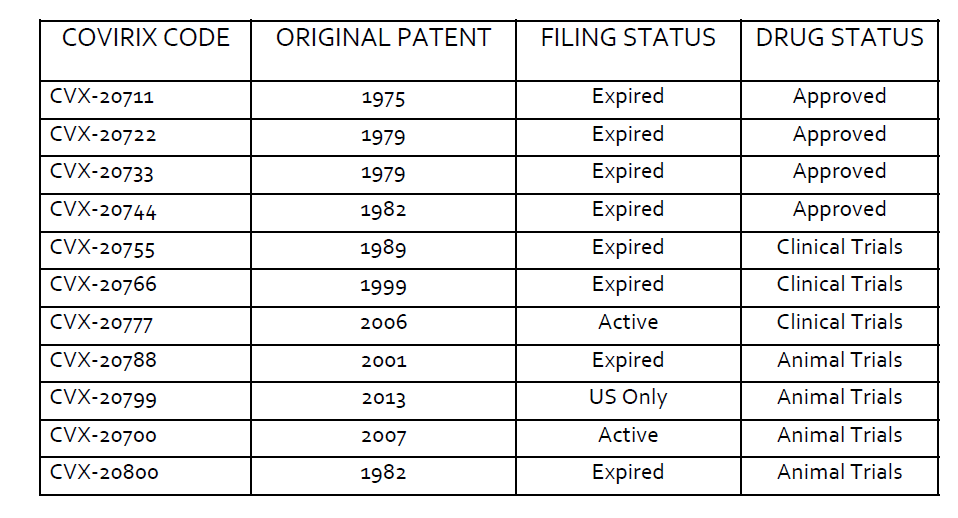
A search of the CAS database (the worlds largest chemical database) on the 22nd of July 2021 indicates that the approved drugs claimed in our antivirals application have expired and the originators can therefore no longer have any claims to the chemical entities/drugs. A summary of the search results are set out below.